RWJ-51204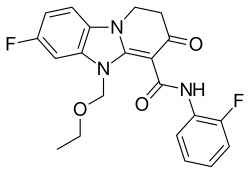 |
|
5-ethoxymethyl-7-fluoro-3-oxo-1,2,3,5-tetrahydrobenzo[4,5] imidazo[1,2a]pyridine-4-N-(2-fluorophenyl)carboxamide
|
| CAS Number | |
|---|
| PubChem CID | |
|---|
| ChemSpider | |
|---|
| UNII | |
|---|
| ChEMBL | |
|---|
| CompTox Dashboard (EPA) | |
|---|
|
| Formula | C21H19F2N3O3 |
|---|
| Molar mass | 399.398 g·mol−1 |
|---|
| 3D model (JSmol) | |
|---|
Fc1ccccc1NC(=O)C=3C(=O)CCN4c2c(cc(F)cc2)N(C=34)COCC
|
InChI=1S/C21H19F2N3O3/c1-2-29-12-26-17-11-13(22)7-8-16(17)25-10-9-18(27)19(21(25)26)20(28)24-15-6-4-3-5-14(15)23/h3-8,11H,2,9-10,12H2,1H3,(H,24,28)  Y YKey:VQOQDABVGWLROX-UHFFFAOYSA-N  Y Y
|
 N N Y (what is this?) (verify) Y (what is this?) (verify) |
RWJ-51204 is an anxiolytic drug used in scientific research. It has similar effects to benzodiazepine drugs, but is structurally distinct and so is classed as a nonbenzodiazepine anxiolytic.
RWJ-51204 is a nonselective partial agonist at GABAA receptors.[1] It produces primarily anxiolytic effects at low doses, with sedative, ataxia and muscle relaxant effects only appearing at some 20x the effective anxiolytic dose.[2] It was discovered by researchers at the pharmaceutical company Johnson & Johnson,[3][4] but its development has been discontinued.
References
- ^ Atack JR (August 2003). "Anxioselective compounds acting at the GABA(A) receptor benzodiazepine binding site". Current Drug Targets. CNS and Neurological Disorders. 2 (4): 213–32. doi:10.2174/1568007033482841. PMID 12871032.
- ^ Dubinsky B, Vaidya AH, Rosenthal DI, Hochman C, Crooke JJ, DeLuca S, DeVine A, Cheo-Isaacs CT, Carter AR, Jordan AD, Reitz AB, Shank RP (November 2002). "5-ethoxymethyl-7-fluoro-3-oxo-1,2,3,5-tetrahydrobenzo[4,5]imidazo[1,2a]pyridine-4-N-(2-fluorophenyl)carboxamide (RWJ-51204), a new nonbenzodiazepine anxiolytic". The Journal of Pharmacology and Experimental Therapeutics. 303 (2): 777–90. doi:10.1124/jpet.102.036954. PMID 12388665. S2CID 23880756.
- ^ US 5817668, Reitz AB, Jordan AD, Sanfilippo PJ, Vavouyios-Smith A, issued 6 October 1998, assigned to Ortho Pharma Corp
- ^ Cohen JH, Maryanoff CA, Stefanik SM, Sorgi KL, Villani FJ (1999). "Process research for the synthesis of RWJ-51204, a novel anxiolytic agent". Organic Process Research & Development. 3 (4): 260–265. doi:10.1021/op990182l.
|
|---|
| 5-HT1ARTooltip 5-HT1A receptor agonists | |
|---|
| GABAARTooltip GABAA receptor PAMsTooltip positive allosteric modulators | |
|---|
Gabapentinoids
(α2δ VDCC blockers) | |
|---|
| Antidepressants | |
|---|
Sympatholytics
(Antiadrenergics) |
- Alpha-1 blockers (e.g., prazosin)
- Alpha-2 agonists (e.g., clonidine, dexmedetomidine, guanfacine)
- Beta blockers (e.g., propranolol, atenolol, betaxolol, nadolol, oxprenolol, pindolol)
|
|---|
| Others | |
|---|
|
|
|---|
| Alcohols | |
|---|
| Barbiturates | |
|---|
| Benzodiazepines | |
|---|
| Carbamates | |
|---|
| Flavonoids |
- 6-Methylapigenin
- Ampelopsin (dihydromyricetin)
- Apigenin
- Baicalein
- Baicalin
- Catechin
- EGC
- EGCG
- Hispidulin
- Linarin
- Luteolin
- Rc-OMe
- Skullcap constituents (e.g., baicalin)
- Wogonin
|
|---|
| Imidazoles | |
|---|
| Kava constituents |
- 10-Methoxyyangonin
- 11-Methoxyyangonin
- 11-Hydroxyyangonin
- Desmethoxyyangonin
- 11-Methoxy-12-hydroxydehydrokavain
- 7,8-Dihydroyangonin
- Kavain
- 5-Hydroxykavain
- 5,6-Dihydroyangonin
- 7,8-Dihydrokavain
- 5,6,7,8-Tetrahydroyangonin
- 5,6-Dehydromethysticin
- Methysticin
- 7,8-Dihydromethysticin
- Yangonin
|
|---|
| Monoureides | |
|---|
| Neuroactive steroids | |
|---|
| Nonbenzodiazepines | |
|---|
| Phenols | |
|---|
| Piperidinediones | |
|---|
| Pyrazolopyridines | |
|---|
| Quinazolinones | |
|---|
| Volatiles/gases | |
|---|
| Others/unsorted |
- 3-Hydroxybutanal
- α-EMTBL
- AA-29504
- Alogabat
- Avermectins (e.g., ivermectin)
- Bromide compounds (e.g., lithium bromide, potassium bromide, sodium bromide)
- Carbamazepine
- Chloralose
- Chlormezanone
- Clomethiazole
- Darigabat
- DEABL
- Deuterated etifoxine
- Dihydroergolines (e.g., dihydroergocryptine, dihydroergosine, dihydroergotamine, ergoloid (dihydroergotoxine))
- DS2
- Efavirenz
- Etazepine
- Etifoxine
- Fenamates (e.g., flufenamic acid, mefenamic acid, niflumic acid, tolfenamic acid)
- Fluoxetine
- Flupirtine
- Hopantenic acid
- KRM-II-81
- Lanthanum
- Lavender oil
- Lignans (e.g., 4-O-methylhonokiol, honokiol, magnolol, obovatol)
- Loreclezole
- Menthyl isovalerate (validolum)
- Monastrol
- Nicotinic acid
- Nicotinamide
- Org 25,435
- Phenytoin
- Propanidid
- Retigabine (ezogabine)
- Safranal
- Seproxetine
- Stiripentol
- Sulfonylalkanes (e.g., sulfonmethane (sulfonal), tetronal, trional)
- Terpenoids (e.g., borneol)
- Topiramate
- Valerian constituents (e.g., isovaleric acid, isovaleramide, valerenic acid, valerenol)
|
|---|
See also: Receptor/signaling modulators • GABA receptor modulators • GABA metabolism/transport modulators |
