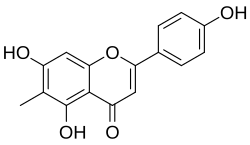
6-Methylapigenin
Names
IUPAC name
5,7-Dihydroxy-2-(4-hydroxyphenyl)-6-methylchromen-4-one
Identifiers
CAS Number
3D model (JSmol)
ChEBI
ChemSpider
Key: ZLGRXDWWYMFIGI-UHFFFAOYSA-N
InChI=1S/C16H12O5/c1-8-11(18)6-14-15(16(8)20)12(19)7-13(21-14)9-2-4-10(17)5-3-9/h2-7,17-18,20H,1H3
CC1=C(C2=C(C=C1O)OC(=CC2=O)C3=CC=C(C=C3)O)O
Properties
Chemical formula
C 16 H 12 O 5
Molar mass
284.267 g·mol−1
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
6-Methylapigenin is a naturally occurring flavonoid and a derivative of apigenin. It has activity at GABAA receptors as a positive modulator .
Natural occurrence
6-Methylapigenin can be found in multiple plants, such as Valeriana officinalis , Valeriana jatamansi , and Picea neoveitchii .[ 1]
Biological activity
6-Methylapigenin binds to the GABAA receptor on the benzodiazepine binding site. This compound possesses anxiolytic effects. In a mouse model, it is also able to potentiate sleep induced by hesperidin, another flavonoid.[ 2] [ 3] benzodiazepines , it can therefore be classed as a nonbenzodiazepine .
References
^ PubChem. "6-Methylapigenin" . pubchem.ncbi.nlm.nih.gov . Retrieved 2024-02-04 . ^ Fernández, Sebastián P.; Wasowski, Cristina; Paladini, Alejandro C.; Marder, Mariel (2005-04-11). "Synergistic interaction between hesperidin, a natural flavonoid, and diazepam". European Journal of Pharmacology . 512 (2– 3): 189– 198. doi:10.1016/j.ejphar.2005.02.039 . ISSN 0014-2999 . PMID 15840404 . ^ Marder, Mariel; Viola, Haydeé; Wasowski, Cristina; Fernández, Sebastián; Medina, Jorge H.; Paladini, Alejandro C. (2003). "6-methylapigenin and hesperidin: new valeriana flavonoids with activity on the CNS". Pharmacology, Biochemistry, and Behavior . 75 (3): 537– 545. doi:10.1016/s0091-3057(03)00121-7 . ISSN 0091-3057 . PMID 12895671 . S2CID 37559366 .
Alcohols Barbiturates Benzodiazepines Carbamates Flavonoids
Ampelopsin (dihydromyricetin)
Apigenin
Baicalein
Baicalin
Catechin
EGC
EGCG Hispidulin
Linarin
Luteolin
Rc-OMe
Skullcap constituents (e.g., baicalin)
Wogonin
Imidazoles Kava constituents
10-Methoxyyangonin
11-Methoxyyangonin
11-Hydroxyyangonin
Desmethoxyyangonin 11-Methoxy-12-hydroxydehydrokavain
7,8-Dihydroyangonin
Kavain
5-Hydroxykavain
5,6-Dihydroyangonin
7,8-Dihydrokavain
5,6,7,8-Tetrahydroyangonin
5,6-Dehydromethysticin
Methysticin 7,8-Dihydromethysticin
Yangonin Monoureides Neuroactive steroids Nonbenzodiazepines Phenols Piperidinediones Pyrazolopyridines Quinazolinones Volatiles /gases Others/unsorted
3-Hydroxybutanal
α-EMTBL
AA-29504
Alogabat Avermectins (e.g., ivermectin )
Bromide compounds (e.g., lithium bromide, potassium bromide , sodium bromide)Carbamazepine Chloralose
Chlormezanone Clomethiazole Darigabat DEABL Deuterated etifoxine Dihydroergolines (e.g., dihydroergocryptine , dihydroergosine, dihydroergotamine , ergoloid (dihydroergotoxine) )DS2
Efavirenz Etazepine Etifoxine Fenamates (e.g., flufenamic acid , mefenamic acid , niflumic acid , tolfenamic acid )
Fluoxetine Flupirtine Hopantenic acid KRM-II-81 Lanthanum
Lavender oil Lignans (e.g., 4-O-methylhonokiol, honokiol, magnolol, obovatol)
Loreclezole Menthyl isovalerate (validolum) Monastrol Nicotinic acid Nicotinamide Org 25,435 Phenytoin Propanidid Retigabine (ezogabine) Safranal
Seproxetine Stiripentol Sulfonylalkanes (e.g., sulfonmethane (sulfonal) , tetronal , trional )
Terpenoids (e.g., borneol)
Topiramate Valerian constituents (e.g., isovaleric acid , isovaleramide, valerenic acid , valerenol) See also: Receptor/signaling modulators • GABA receptor modulators • GABA metabolism/transport modulators