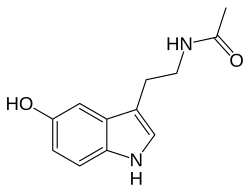
N -Acetylserotonin
Names
Preferred IUPAC name
N -[2-(5-Hydroxy-1H -indol-3-yl)ethyl]acetamide
Other names
N -Acetyl-5-hydroxytryptamineN -Acetyl-5-HT
Identifiers
CAS Number
3D model (JSmol)
ChEBI
ChEMBL
ChemSpider
DrugBank
ECHA InfoCard
100.013.560
MeSH
N -Acetylserotonin N -Acetylserotonin
UNII
InChI=1S/C12H14N2O2/c1-8(15)13-5-4-9-7-14-12-3-2-10(16)6-11(9)12/h2-3,6-7,14,16H,4-5H2,1H3,(H,13,15)
Y Key: MVAWJSIDNICKHF-UHFFFAOYSA-N
Y InChI=1/C12H14N2O2/c1-8(15)13-5-4-9-7-14-12-3-2-10(16)6-11(9)12/h2-3,6-7,14,16H,4-5H2,1H3,(H,13,15)
Key: MVAWJSIDNICKHF-UHFFFAOYAX
CC(=O)NCCC1=CNC2=C1C=C(C=C2)O
Properties
Chemical formula
C 12 H 14 N 2 O 2
Molar mass
218.256 g·mol−1
Density
1.268 g/mL
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
N -AcetylserotoninNAS ), also known as normelatonin , is a naturally occurring chemical intermediate in the endogenous production of melatonin from serotonin .[ 1] [ 2] biological activity in its own right, including acting as a melatonin receptor agonist , an agonist of the TrkB, and having antioxidant effects.
Biological function
Like melatonin, NAS is an agonist at the melatonin receptors MT1 , MT2 , and MT3 , and may be considered to be a neurotransmitter .[ 3] [ 4] [ 5] [ 6] brain where serotonin and melatonin are not, suggesting that it may have unique central duties of its own instead of merely functioning as a precursor in the synthesis of melatonin.[ 3] [ 7] [ 8] [ 8]
TrkB receptor
NAS has been shown to act as a potent TrkB receptor agonist, while serotonin and melatonin do not.[ 3]
progenitor cells (NPC)s, blockage of TrkB abolished this effect suggesting that it is TrkB-dependent.[ 9] [ 9] [ 7]
Antioxidant properties
NAS acts as a potent antioxidant , NAS effectiveness as an anti-oxidant has been found to be different depending on the experimental model used, it has been described as being between 5 and 20 times more effect than melatonin at protecting against oxidant damage.[ 10] [ 11] ROS in peripheral blood lymphocytes and to exhibit anti-oxidant effects against t-butylated hydroperoxide- and diamide-induced ROS.[ 12] [ 13]
Anti-inflammatory effects
NAS has been reported to have anti-inflammatory effects. NAS has been shown to inhibit LPS -stimulated production of the proinflammatory cytokine TNF-alpha in differentiated THP-1-derived human monocytes.[ 14]
Miscellaneous
NAS may play a role in the antidepressant effects of selective serotonin reuptake inhibitors (SSRIs) and monoamine oxidase inhibitors (MAOIs).[ 3] fluoxetine and the MAO-A inhibitor clorgyline upregulate AANAT indirectly through serotonergic mechanisms and thereby increase NAS levels after chronic administration, and this correlates with the onset of their antidepressant effects.[ 3] [ 15] [ 3] knockout mice display significantly greater immobility times versus control mice in animal models of depression .[ 3]
Through a currently unidentified mechanism, NAS may be the cause of the orthostatic hypotension seen with clinical treatment of MAOIs.[ 15] [ 16] blood pressure in rodents, and pinealectomy (the pineal gland being a major site of NAS and melatonin synthesis) abolishes the hypotensive effects of clorgyline .[ 15] [ 16]
Biochemistry
NAS is produced from serotonin by the enzyme aralkylamine N -acetyltransferase (AANAT) and is converted to melatonin by acetylserotonin O -methyltransferase (ASMT).
NAS is able to penetrate the blood–brain barrier , unlike serotonin.[ 17]
See also
Tropomyosin receptor kinase B § Agonists
N-Acetyldopamine
References
^ AXELROD J, WEISSBACH H (April 1960). "Enzymatic O-methylation of N-acetylserotonin to melatonin". Science . 131 (3409): 1312. Bibcode:1960Sci...131.1312A . doi:10.1126/science.131.3409.1312 . PMID 13795316 . S2CID 22341451 . ^ WEISSBACH H, REDFIELD BG, AXELROD J (September 1960). "Biosynthesis of melatonin: enzymic conversion of serotonin to N-acetylserotonin". Biochimica et Biophysica Acta . 43 : 352– 3. doi:10.1016/0006-3002(60)90453-4 . PMID 13784117 . ^ a b c d e f g Jang SW, Liu X, Pradoldej S, et al. (February 2010). "N-acetylserotonin activates TrkB receptor in a circadian rhythm" . Proceedings of the National Academy of Sciences of the United States of America . 107 (8): 3876– 81. Bibcode:2010PNAS..107.3876J . doi:10.1073/pnas.0912531107 2840510 20133677 . ^ Zhao H, Poon AM, Pang SF (March 2000). "Pharmacological characterization, molecular subtyping, and autoradiographic localization of putative melatonin receptors in uterine endometrium of estrous rats". Life Sciences . 66 (17): 1581– 91. doi:10.1016/S0024-3205(00)00478-1 . PMID 11261588 . ^ Nonno R, Pannacci M, Lucini V, Angeloni D, Fraschini F, Stankov BM (July 1999). "Ligand efficacy and potency at recombinant human MT2 melatonin receptors: evidence for agonist activity of some mt1-antagonists" . British Journal of Pharmacology . 127 (5): 1288– 94. doi:10.1038/sj.bjp.0702658 . PMC 1566130 10455277 . ^ Paul P, Lahaye C, Delagrange P, Nicolas JP, Canet E, Boutin JA (July 1999). "Characterization of 2-[125I]iodomelatonin binding sites in Syrian hamster peripheral organs" The Journal of Pharmacology and Experimental Therapeutics . 290 (1): 334– 40. doi:10.1016/S0022-3565(24)34904-3 . PMID 10381796 . ^ a b Tosini G., Ye K. & Iuvone PM (2012). "Neuroprotection, neurogenesis, and the sleepy brain" . Neuroscientist . 18 (6): 645– 653. doi:10.1177/1073858412446634 . PMC 3422380 22585341 . ^ a b Oxenkrug G. & Ratner R. (2012). "N-acetylserotonin and aging-associated cognitive impairment and depression" . Aging Dis . 3 (4): 330– 338. PMC 3501368 23185714 . ^ a b Sompol P.; Liu X.; Baba K.; Paul KN.; Tosini G.; Iuvone PM.; Ye K. (2011). "N-acetylserotonin promotes hippocampal neuroprogenitor cell proliferation in sleep-deprived mice" . Proc. Natl. Acad. Sci. U.S.A . 108 (21): 8844– 9. Bibcode:2011PNAS..108.8844S . doi:10.1073/pnas.1105114108 3102377 21555574 . ^ Oxenkrug G (2005). "Antioxidant effects of N-acetylserotonin: possible mechanisms and clinical implications". Ann. N. Y. Acad. Sci . 1053 (1): 334– 47. Bibcode:2005NYASA1053..334O . doi:10.1111/j.1749-6632.2005.tb00042.x . PMID 16179540 . S2CID 94273958 . ^ Gavazza MB.; Català A. (2004). "Protective effect of N-acetyl-serotonin on the nonenzymatic lipid peroxidation in rat testicular microsomes and mitochondria". J. Pineal Res . 37 (3): 153– 60. doi:10.1111/j.1600-079x.2004.00150.x . PMID 15357659 . S2CID 6974587 . ^ Wölfler A.; Abuja PM.; Schauenstein K.; Liebmann PM. (1999). "N-acetylserotonin is a better extra- and intracellular antioxidant than melatonin". FEBS Lett . 449 (2– 3): 206– 10. Bibcode:1999FEBSL.449..206W . doi:10.1016/s0014-5793(99)00435-4 . PMID 10338133 . S2CID 32077728 . ^ Peter Klemm; Markus Hecker; Hannelore Stockhausen; Chin Chen Wu; Christoph Thiemermann (Aug 1995). "Inhibition by N-acetyl-5-hydroxytryptamine of nitric oxide synthase expression in cultured cells and in the anaesthetized rat" . Br J Pharmacol . 115 (7): 1175– 1181. doi:10.1111/j.1476-5381.1995.tb15021.x . PMC 1908794 7582541 . ^ Perianayagam MC.; Oxenkrug GF.; Jaber BL. (2005). "Immune-modulating effects of melatonin, N-acetylserotonin, and N-acetyldopamine". Ann. N. Y. Acad. Sci . 1053 : 386– 93. doi:10.1111/j.1749-6632.2005.tb00046.x . PMID 16179544 . S2CID 592935 . ^ a b c Oxenkrug GF (1999). "Antidepressive and antihypertensive effects of MAO-A inhibition: role of N-acetylserotonin. A review". Neurobiology (Budapest, Hungary) . 7 (2): 213– 24. PMID 10591054 . ^ a b Oxenkrug GF (1997). "[N-acetylserotonin and hypotensive effect of MAO-A inhibitors]". Voprosy Meditsinskoi Khimii (in Russian). 43 (6): 522– 6. PMID 9503569 . ^ "N-Acetyl Serotonin" . DrugBank.
Food antioxidants Fuel antioxidants
Butylated hydroxyanisole
Butylated hydroxytoluene 2,6-Di-tert -butylphenol
1,2-Diaminopropane
2,4-Dimethyl-6-tert -butylphenol
Ethylenediamine Measurements
Folin method
ORAC
TEAC
FRAP
Angiopoietin
Agonists: Angiopoietin 1Angiopoietin 4 Antagonists: Angiopoietin 2Angiopoietin 3 Kinase inhibitors: AltiratinibCE-245677
Rebastinib CNTF EGF (ErbB)
EGF(ErbB1/HER1)
Agonists : AmphiregulinBetacellulin
EGF (urogastrone)
Epigen
Epiregulin
Heparin-binding EGF-like growth factor (HB-EGF)
Murodermin Nepidermin Transforming growth factor alpha (TGFα) ErbB2/HER2 ErbB3/HER3
Agonists: Neuregulins (heregulins) (1, 2, 6 (neuroglycan C)) ErbB4/HER4
Agonists: BetacellulinEpigen
Heparin-binding EGF-like growth factor (HB-EGF)
Neuregulins (heregulins) (1, 2, 3, 4, 5 (tomoregulin, TMEFF))
FGF
FGFR1
Agonists: ErsoferminFGF (1, 2 (bFGF), 3, 4, 5, 6, 8, 10 (KGF2), 20)
Repifermin
Selpercatinib Trafermin Velafermin FGFR2
Agonists: ErsoferminFGF (1, 2 (bFGF), 3, 4, 5, 6, 7 (KGF ), 8, 9, 10 (KGF2), 17, 18, 22)
Palifermin Repifermin
Selpercatinib Sprifermin
Trafermin Antibodies: AprutumabAprutumab ixadotin FGFR3 FGFR4
Agonists: ErsoferminFGF (1, 2 (bFGF), 4, 6, 8, 9, 19 )
Trafermin Unsorted
HGF (c-Met) IGF
IGF-1
Kinase inhibitors: BMS-754807Linsitinib NVP-ADW742
NVP-AEW541
OSl-906 IGF-2
Agonists : Insulin-like growth factor-2 (somatomedin A)Antibodies: Dusigitumab Xentuzumab (against IGF-1 and IGF-2) Others
Binding proteins: IGFBP (1, 2, 3, 4, 5, 6, 7)Cleavage products/derivatives with unknown target: Glypromate (GPE, (1-3)IGF-1)Trofinetide
LNGF (p75NTR )
Aptamers: Against NGF: RBM-004Decoy receptors: LEVI-04 (p75NTR -Fc) PDGF
Agonists: Becaplermin Platelet-derived growth factor (A, B, C, D) RET (GFL)
GFRα1
Agonists: Glial cell line-derived neurotrophic factor (GDNF)Liatermin GFRα2
Agonists: Neurturin (NRTN) GFRα3 GFRα4
Agonists: Persephin (PSPN) Unsorted
Kinase inhibitors: Agerafenib
SCF (c-Kit) TGFβ Trk
TrkA
Negative allosteric modulators: VM-902AAptamers: Against NGF: RBM-004Decoy receptors: ReN-1820 (TrkAd5) TrkB
Agonists: 3,7-DHF3,7,8,2'-THF
4'-DMA-7,8-DHF 7,3'-DHF
7,8-DHF 7,8,2'-THF
7,8,3'-THF Amitriptyline BDNF BNN-20 Deoxygedunin Deprenyl Diosmetin
DMAQ-B1
HIOC
LM22A-4 NT-3
NT-4
Norwogonin (5,7,8-THF) R7 R13 TDP6 TrkC
VEGF
Agonists: Placental growth factor (PGF)Ripretinib Telbermin
VEGF (A, B, C, D (FIGF)) Others
Additional growth factors: Adrenomedullin Colony-stimulating factors (see here instead)
Connective tissue growth factor (CTGF)
Ephrins (A1, A2, A3, A4, A5, B1, B2, B3)
Erythropoietin (see here instead)Glucose-6-phosphate isomerase (GPI; PGI, PHI, AMF)
Glia maturation factor (GMF)
Hepatoma-derived growth factor (HDGF)
Interleukins /T-cell growth factors (see here instead)Leukemia inhibitory factor (LIF) Macrophage-stimulating protein (MSP; HLP, HGFLP)
Midkine (NEGF2)
Migration-stimulating factor (MSF; PRG4)
Oncomodulin
Pituitary adenylate cyclase-activating peptide (PACAP)
Pleiotrophin
Renalase
Thrombopoietin (see here instead)
Wnt signaling proteins Additional growth factor receptor modulators: Cerebrolysin (neurotrophin mixture)
MT1 Tooltip Melatonin receptor 1 MT2 Tooltip Melatonin receptor 2 Unsorted
Agonists: 2-Phenylmelatonin5-Methoxyluzindole
6-Chloromelatonin
6-Fluoromelatonin
6-Methoxymelatonin
6,7-Dichloro-2-methylmelatonin
8-M-PDOT
AMMTC
GR-135,531 (of MT3 Tooltip melatonin receptor 1C )
N-Acetyltryptamine
N-Butanoylmelatonin
N-Propionylmelatonin
S-24268
S-25150 Antagonists: 4-P-ADOT4-P-PDOT
DH-97
N-Acetyltryptamine (of MT3 )
Prazosin (of MT3 )S-20928
S-22153
S-24601
S-25567
S-26131
See also: Receptor/signaling modulatorsAdrenergics Dopaminergics Serotonergics Monoamine metabolism modulators
Tryptamines 4-Hydroxytryptamines 5-Hydroxy- and5-methoxytryptamines
2-Methyl-5-HT 4-HO-5-MeO-T 4-F-5-MeO-DMT 4,5-DHP-DMT 4,5-DHT 4,5-MDO-DMT 4,5-MDO-DiPT 5-BT 5-Ethoxy-DMT
5-HO-DET 5-HO-DiPT 5-HO-NiPT
5-HO-DPT 5-HTP (oxitriptan )5-MeO-2-TMT 5-MeO-34MPEMT
5-MeO-7,N ,N -TMT 5-MeO-DALT 5-MeO-DBT 5-MeO-DET 5-MeO-DiPT 5-MeO-DMT (N ,N ,O -TMS; O -methylbufotenine) 5-MeO-DPT 5-MeO-EiPT 5-MeO-EPT 5-MeO-MALT 5-MeO-MET 5-MeO-MiPT 5-MeO-NET 5-MeO-NiPT 5-MeO-NMT (O ,N -DMS) 5-MeO-PiPT 5-MeO-NBpBrT 5-MeO-T (5-MT; mexamine; O -methylserotonin) 5-MeO-T-NBOMe 5-MT-NB3OMe
5-NOT
5,6-DHT 5,6-MDO-DiPT 5,6-MDO-DMT 5,6-MDO-MiPT 5,6-MeO-MiPT 5,7-DHT
Arachidonoyl serotonin ASR-3001 (5-MeO-iPALT) BAB
Benanserin (BAS; SQ-4788) BGC20-761 Bufotenidine (5-HTQ; N ,N ,N -TMS)
Bufotenin (5-HO-DMT; N ,N -DMS; mappine) Bufoviridine (5-SO-DMT)
CP-132,484 Cqd 280
Cqd 285
Cqdd 280
Donitriptan EMDT (2-Et-5-MeO-DMT) HIOC
Indorenate (TR-3369)
Isamide (N -CA-5-MT) L-741604 MS-245 N -DEAOP-5-MeO-NETN -DEAOP-5-MeO-NMTN -Feruloylserotonin (moschamine)Norbufotenin (5-HO-NMT; NMS) O -Acetylbufotenine (5-AcO-DMT)O -Pivalylbufotenine (5-(t -BuCO)-DMT)Psilomethoxin (4-HO-5-MeO-DMT) Psilomethoxybin (4-PO-5-MeO-DMT) Serotonin (5-HT) N -Acetyltryptaminesα-Alkyltryptamines
5-Hydroxy- and 5-alkoxy-α-alkyltryptamines: 1-Pr-5-MeO-AMT 5-Allyloxy-AMT 5-Ethoxy-αMT 5-iPrO-αMT
5-MeO-αET 5-MeO-αMT (α,O -DMS; Alpha-O) α-Methyl-5-HTP α-Methylmelatonin α-Methylserotonin (5-HO-αMT; α-Me-5-HT) α,N ,O -TMS (5-MeO-α,N -DMT) α,N ,N ,O -TeMS (5-MeO-α,N ,N -TMT) AL-37350A (4,5-DHP-αMT) BW-723C86 Cyclized tryptamines
Barettin
Cyclic 3-OHM
Ergolines and lysergamides (e.g., LSD )Harmala alkaloidsβ-carbolines (e.g., 5-methoxyharmalan, 6-MeO-THH , 6-methoxyharmalan , 9-Me-BC, β-carboline (norharman) , fenharmane , harmaline , harmalol, harmane, harmine , LY-266,097 , pinoline, tetrahydroharmine , tryptoline)Iboga alkaloidsibogaine , ibogamine , noribogaine , tabernanthine )Ibogalogs (e.g., catharanthalog , fluorogainalog, ibogainalog , ibogaminalog (DM-506) , LS-22925, noribogainalog , noribogaminalog , PHA-57378 , PNU-22394 , tabernanthalog )Imidazolylindoles (e.g., AGH-107 , AGH-192 , AH-494)Metralindole Partial ergolines and lysergamides (e.g., NDTDI , RU-27849 , RU-28251 , RU-28306 , FHATHBIN , LY-178210 , Bay R 1531 (LY-197206) , LY-293284 , 10,11-seco-LSD , 10,11-secoergoline (α,N -Pip-T) , CT-5252 )Pertines (e.g., alpertine , milipertine , oxypertine , solypertine )Piperidinylethylindoles (e.g., pip-T , indolylethylfentanyl)Pyrrolidinylethylindoles (e.g., pyr-T , 4-HO-pyr-T , 5-MeO-pyr-T , 4-F-5-MeO-pyr-T )Pyrrolidinylmethylindoles (e.g., MPMI , 4-HO-MPMI (lucigenol) , 5F-MPMI , 5-MeO-MPMI , CP-122288 , CP-135807 , eletriptan )Tetrahydrocarbazolamines (e.g., ciclindole , flucindole , frovatriptan , LY-344864 , ramatroban )Tetrahydropyridinylindoles (e.g., RS134-49 , RU-28253 )Tetrahydropyrroloquinolines (e.g., bufothionine, O -methylnordehydrobufotenineYohimbans (e.g., yohimbine , rauwolscine , spegatrine, corynanthine , ajmalicine , reserpine , deserpidine , rescinnamine ) Isotryptamines Related compounds
2-Azapsilocin
4-Aza-5-MeO-DPT
5-Aza-4-MeO-DiPT
5-HIAA
5-HIAL 5-HITCA
5-MIAL
7-Aza-5-MeO-DiPT
α-Carboline
γ-Carbolines (pyridoindoles) (e.g., γ-carboline, alosetron , gevotroline , latrepirdine , lurosetron , mebhydrolin , tiflucarbine )
Amedalin Benzindopyrine
Benzofurans (e.g., 3-APB , 5-MeO-DiBF , BPAP , 3-F-BPAP , dimemebfe , mebfap , oxa-noribogaine )Benzothiophenes (e.g., 3-APBT )Carmoxirole CT-4436
Daledalin Gramine
Histamine
I-32
IAL
IN-399
Indazolethylamines (e.g., AL-34662 , AL-38022A , O -methyl-AL-34662VU6067416 , YM-348 )Indenylethylamines (e.g., C-DMT )Indolizinylethylamines (e.g., TACT908 (2ZEDMA) , 1ZP2MA , 1Z2MAP1O )Indolylaminopropanes (e.g., 1-API, 2-API, 4-API, 5-API (5-IT; PAL-571) , 6-API (6-IT) , 7-API)Iprindole Masupirdine Medmain
Molindone Non-tryptamine triptans (e.g., avitriptan , LY-334370 , naratriptan )
Ondansetron Oxazinopyridoindoles (e.g., IHCH-8134)Phenethylamines (e.g., phenethylamine , amphetamine )Piperidinylindoles (e.g., BRL-54443 , LY-334370 , naratriptan , sertindole , SN-22 )Pirlindole Pyridinylindoles (e.g., tepirindole )Pyridopyrroloquinoxalines (e.g., IHCH-7113 , IHCH-7079 , IHCH-7086 , lumateperone , deulumateperone , ITI-1549 )Pyrrolylethylamines (e.g., 2-pyrrolylethylamine (NEA) , 3-pyrrolylethylamine (3-NEA) , 3-pyrrolylpropylamine )
Pyrrolopyridinylethylamines (e.g., WAY-208466 )Quinolinylethylamines (e.g., mefloquine )Ro60-0213
Selisistat Tetrahydropyridinylindoles (e.g., EMD-386088 , LY-367265 , RU-24,969 )Tetrahydropyridinylpyrrolopyridines (e.g., (R )-69 (3IQ) , (R )-70, CP-94253 )Tetrindole Tipindole Zilpaterol (RU-42173)
See also: PhenethylaminesErgolines and lysergamides Psychedelics