JTE 7-31
Names
Preferred IUPAC name
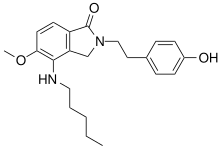
2-[2-(4-Hydroxyphenyl)ethyl]-5-methoxy-4-(pentylamino)-2,3-dihydro-1H -isoindol-1-one
Identifiers
CAS Number
3D model (JSmol)
ChemSpider
UNII
InChI=1S/C22H28N2O3/c1-3-4-5-13-23-21-19-15-24(14-12-16-6-8-17(25)9-7-16)22(26)18(19)10-11-20(21)27-2/h6-11,23,25H,3-5,12-15H2,1-2H3
Y Key: FMUMUYFMLZGXJR-UHFFFAOYSA-N
Y InChI=1/C22H28N2O3/c1-3-4-5-13-23-21-19-15-24(14-12-16-6-8-17(25)9-7-16)22(26)18(19)10-11-20(21)27-2/h6-11,23,25H,3-5,12-15H2,1-2H3
Key: FMUMUYFMLZGXJR-UHFFFAOYAA
Oc1ccc(cc1)CCN3Cc2c(ccc(OC)c2NCCCCC)C3=O
Properties
Chemical formula
C22 H28 N2 O3
Molar mass
368.469 g/mol
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
JTE 7-31 is a selective cannabinoid receptor agonist invented by Japan Tobacco.[ 1] [ 2] 2 agonist, but still retains appreciable affinity at CB1 , with a Ki of 0.088nM at CB2 vs 11nM at CB1 .[ 3]
Legality
JTE 7-31 is illegal in Alabama.[ 4]
See also
References
^ WO patent 1997/029079 , Inaba T, Kaya T, Iwamura H, "Novel compounds and pharmaceutical use thereof", granted 1997-14-08 ^ US patent 6017919 , Inaba T, Kaya T, Iwamura H, "Compounds and pharmaceutical use thereof", granted 2000-01-25 ^ Han S, Zhang FF, Qian HY, Chen LL, Pu JB, Xie X, Chen JZ (March 2015). "Design, syntheses, structure-activity relationships and docking studies of coumarin derivatives as novel selective ligands for the CB2 receptor". European Journal of Medicinal Chemistry . 93 : 16– 32. doi:10.1016/j.ejmech.2015.01.054 . PMID 25644673 . ^ "Alabama Senate Bill SB 333: Controlled Substances" (PDF) .
External links
Phytocannabinoids
Cannabibutols Cannabichromenes Cannabicyclols Cannabidiols Cannabielsoins Cannabigerols Cannabiphorols Cannabinols
CBN
CBNA
CBN-C1
CBN-C2
CBN-C4
CBNM CBND
CBNP
CBVD
Cannabitriols Cannabivarins Delta-3-tetrahydrocannabinols Delta-4-tetrahydrocannabinols Delta-7-tetrahydrocannabinols Delta-8-tetrahydrocannabinols Delta-9-tetrahydrocannabinols Delta-10-Tetrahydrocannabinols Delta-11-Tetrahydrocannabinols Miscellaneous cannabinoids Active metabolites
Endocannabinoids Synthetic
Classical cannabinoids Non-classical Adamantoylindoles Benzimidazoles Benzoylindoles
1-Butyl-3-(2-methoxybenzoyl)indole
1-Butyl-3-(4-methoxybenzoyl)indole
1-Pentyl-3-(2-methoxybenzoyl)indole
AM-630
AM-679
AM-694
AM-1241
AM-2233 GW-405,833 (L-768,242)
Pravadoline RCS-4 WIN 54,461 Cyclohexylphenols Eicosanoids
AM-883 AM-1346
ACEA
ACPA
Methanandamide (AM-356)
O-585
O-689
O-1812 O-1860
O-1861 Indazole-3- Indole-3-carboxamides Indole-3-carboxylates Naphthoylindazoles Naphthoylindoles Naphthoylpyrroles
JWH-030 JWH-031
JWH-032
JWH-033
JWH-036
JWH-044
JWH-045
JWH-145 JWH-146 JWH-147 JWH-150 JWH-156
JWH-243
JWH-244
JWH-245
JWH-246
JWH-292
JWH-293
JWH-307 JWH-308
JWH-309
JWH-346
JWH-347
JWH-348
JWH-363
JWH-364
JWH-365
JWH-366
JWH-367
JWH-368
JWH-369
JWH-370
JWH-371
JWH-372
JWH-373 Naphthylmethylindenes Naphthylmethylindoles
JWH-175 JWH-184
JWH-185
JWH-192
JWH-194
JWH-195
JWH-196
JWH-197
JWH-199 Phenylacetylindoles Pyrazolecarboxamides Tetramethylcyclo- Tetramethylcyclo- Others
Allosteric CBR Tooltip Cannabinoid receptor ligandsEndocannabinoid (inactivation inhibitors) Anticannabinoids (antagonists/inverse